03.15.19
By Behrooz Yadegar, MediSens Wireless, and Carol Whitehurst, Casa Dorinda
A non-randomized group study was sponsored by MediSens Wireless, Inc. and conducted at Casa Dorinda Retirement Community to determine the effectiveness of the WetSens Wearable Sensor Platform in improving the quality of care and life of incontinent adults.
Casa Dorinda Retirement Community is a non-profit continuing care community in Santa Barbara, CA, that provides exceptional standards of care for its residents.
MediSens Wireless, Inc. is a medical device company which has developed a wearable sensor platform which is focused on addressing the unmet needs of incontinence management in extended healthcare facilities around the world. It is projected that more than 50% of residents of extended care facilities use some type of incontinence products.
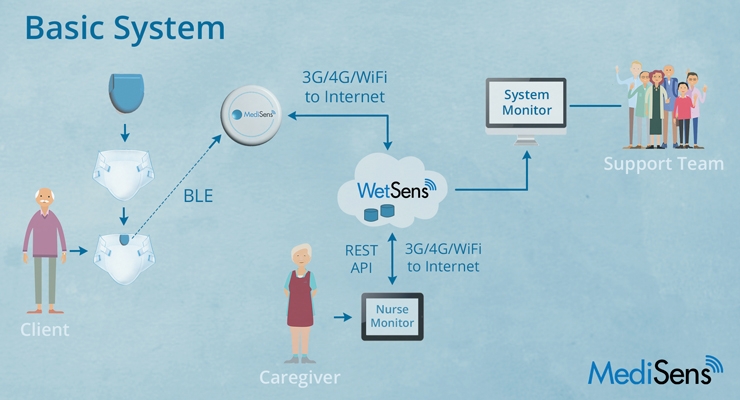
The MediSens WetSens Wearable Sensor Platform (hereafter referred as Abena Nova with MediSens or system) includes an Abena disposable incontinence product with an embedded sensor designed by MediSens, and a reusable WetSens Communications Clip. The WetSens Clip is attached to the sensor product and communicates using Bluetooth Low Energy (BLE) technology. When the sensors detect the presence of urine, the event is sent to the Nurse Monitor used by the caregivers. The caregivers are notified in real time of a wet incontinence product on the resident. This improvement in incontinence care reduces the number of leakages and extends periods of skin dryness which may reduce skin irritation, rash and urinary tract events.
Participant Population
Initially, 16 adult resident participants at Casa Dorinda were enrolled in the study. Of these 16 participants, 15 were female. All participants experience some type of dementia and incontinent events. Three were unenrolled in the first week.
Duration and Data Collection
The study was conducted over six weeks with four different phases.
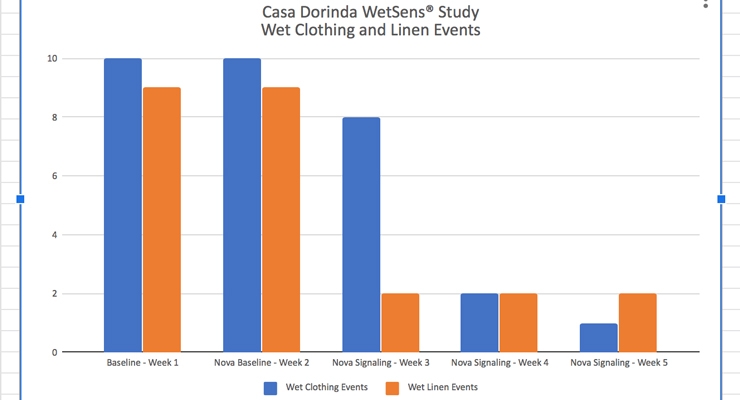
Overall wet linen events were reduced from nine to two events per week over the participant population of 13. This demonstrated a 78% improvement. Overall wet clothing events were reduced from 10 events to one event over the participant population of 13. This demonstrated a 90% improvement.
Additionally, overall incontinence product usage was reduced from an average of six products per participant client per day to five products per participant client per day.
An ”Alert Threshold” was established in the WetSens Platform for the Casa Dorinda study. An “Alert Threshold” is a wetness level programmed for each incontinence product. Once the system determines that the “Alert Threshold” has been reached, it sends the notification to the Nurse Monitor that the incontinence product is wet and requires a change. During week two, Nova Baseline phase, the sending of that notification was turned off so that a baseline could be established to determine the average time a participant resident was in a wet incontinence product prior to the caregiver changing the incontinence product. This time is referred to as the “Response Time.
” The average Response Time established during the Nova Baseline phase was 114 minutes. The average Response Time established during weeks three to five, the Nova Signaling phase, was 83 minutes. The overall response time was reduced by 31 minutes which is a 28% improvement.
With the reduction of Response Time by 31 minutes, each participant resident was drier by an extra 2 hours and 35 minutes per day, referred to as Dry Time. Improvement in Dry Time may aid in the reduction and prevention of skin irritation, rashes, and urinary tract infections.
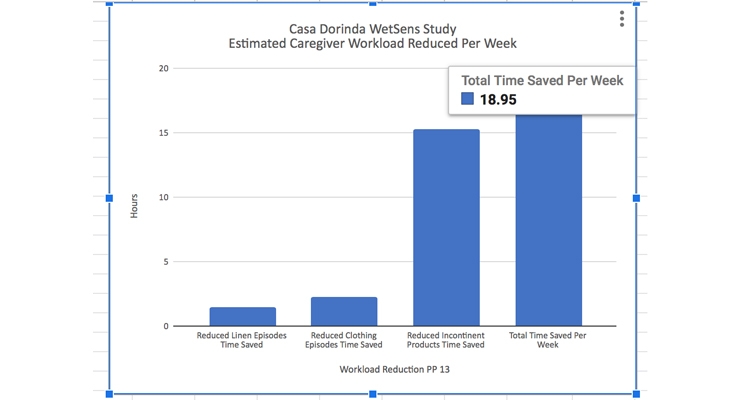
Caregiver work time was reduced significantly based on the reduction of wet linen events, wet clothes events, and number of incontinent products used per week. Using an estimated time of 15 minutes per wet clothing and wet linen events and an estimated time of 10 minutes per diaper change, caregiver work load was reduced by 19 hours per week over the participant population.
Conclusion
The non-randomized group study conducted at Casa Dorinda was successful in demonstrating the effectiveness of the Abena Nova with MediSens in improving the quality of care and life of adults who are incontinent. The collected data (manually within the “Daily Sheet Forms” and via the electronic data collected by MediSens) illustrated significant reduction in Wet Clothes and Linen Change Episodes, Incontinent Product Usage, and improved Response Time. Additionally, Groin Skin Dry Time was increased. A significant improvement in incontinent management was achieved.
Casa Dorinda WetSens Study Reported Outcomes
Some Notable Comments from Caregivers:
“...saved on clothing changes, which helped to prevent agitation in residents”
“...helps to establish a toileting plan by determining a pattern of when the residents void”
“...most residents did not find it intrusive or bothersome”
“...easy product to use”
“...improvement of sleep for the participants”
“...night time wetness checks were unnecessary”
A non-randomized group study was sponsored by MediSens Wireless, Inc. and conducted at Casa Dorinda Retirement Community to determine the effectiveness of the WetSens Wearable Sensor Platform in improving the quality of care and life of incontinent adults.
Casa Dorinda Retirement Community is a non-profit continuing care community in Santa Barbara, CA, that provides exceptional standards of care for its residents.
MediSens Wireless, Inc. is a medical device company which has developed a wearable sensor platform which is focused on addressing the unmet needs of incontinence management in extended healthcare facilities around the world. It is projected that more than 50% of residents of extended care facilities use some type of incontinence products.
The MediSens WetSens Wearable Sensor Platform (hereafter referred as Abena Nova with MediSens or system) includes an Abena disposable incontinence product with an embedded sensor designed by MediSens, and a reusable WetSens Communications Clip. The WetSens Clip is attached to the sensor product and communicates using Bluetooth Low Energy (BLE) technology. When the sensors detect the presence of urine, the event is sent to the Nurse Monitor used by the caregivers. The caregivers are notified in real time of a wet incontinence product on the resident. This improvement in incontinence care reduces the number of leakages and extends periods of skin dryness which may reduce skin irritation, rash and urinary tract events.
Participant Population
Initially, 16 adult resident participants at Casa Dorinda were enrolled in the study. Of these 16 participants, 15 were female. All participants experience some type of dementia and incontinent events. Three were unenrolled in the first week.
Duration and Data Collection
The study was conducted over six weeks with four different phases.
- Week 1 – Baseline Phase. All participants wore the incontinent products which they had used prior to the study and did not use Abena Nova incontinence products. Caregivers documented wetness events and linen and clothing change events on a provided “Daily Sheet Form.” The purpose of this phase is to collect baseline (current) incontinence management practices.
- Week 2 – Nova Baseline. All participants wore the Abena Nova incontinence products with the communications Clip attached; however, remote notifications of wetness events from the platform were turned off. Caregivers documented wetness events and linen and clothing change events on the “Daily Sheet Form.” The purpose of this phase was to collect baseline wetness data using Abena Nova with MediSens based on current incontinent management practices.
- Week 3 to 5 – Nova Signaling. All participants wore the Abena Nova incontinence products with the communications Clip attached. Remote notifications were sent to the Nurse Monitor when a change of incontinence product was required. Data was collected by the platform as well as “Daily Sheet Form.” The purpose of this phase was to introduce and utilize the MediSens Wearable Sensor Platform.
- Week 6 – Data Collection. Data was not collected on the “Daily Sheet Form.” The purpose of this phase was to tabulate the manual data and transition participants out of the clinical study.
Overall wet linen events were reduced from nine to two events per week over the participant population of 13. This demonstrated a 78% improvement. Overall wet clothing events were reduced from 10 events to one event over the participant population of 13. This demonstrated a 90% improvement.
Additionally, overall incontinence product usage was reduced from an average of six products per participant client per day to five products per participant client per day.
An ”Alert Threshold” was established in the WetSens Platform for the Casa Dorinda study. An “Alert Threshold” is a wetness level programmed for each incontinence product. Once the system determines that the “Alert Threshold” has been reached, it sends the notification to the Nurse Monitor that the incontinence product is wet and requires a change. During week two, Nova Baseline phase, the sending of that notification was turned off so that a baseline could be established to determine the average time a participant resident was in a wet incontinence product prior to the caregiver changing the incontinence product. This time is referred to as the “Response Time.
” The average Response Time established during the Nova Baseline phase was 114 minutes. The average Response Time established during weeks three to five, the Nova Signaling phase, was 83 minutes. The overall response time was reduced by 31 minutes which is a 28% improvement.
With the reduction of Response Time by 31 minutes, each participant resident was drier by an extra 2 hours and 35 minutes per day, referred to as Dry Time. Improvement in Dry Time may aid in the reduction and prevention of skin irritation, rashes, and urinary tract infections.
Caregiver work time was reduced significantly based on the reduction of wet linen events, wet clothes events, and number of incontinent products used per week. Using an estimated time of 15 minutes per wet clothing and wet linen events and an estimated time of 10 minutes per diaper change, caregiver work load was reduced by 19 hours per week over the participant population.
Conclusion
The non-randomized group study conducted at Casa Dorinda was successful in demonstrating the effectiveness of the Abena Nova with MediSens in improving the quality of care and life of adults who are incontinent. The collected data (manually within the “Daily Sheet Forms” and via the electronic data collected by MediSens) illustrated significant reduction in Wet Clothes and Linen Change Episodes, Incontinent Product Usage, and improved Response Time. Additionally, Groin Skin Dry Time was increased. A significant improvement in incontinent management was achieved.
Casa Dorinda WetSens Study Reported Outcomes
- Wet clothing events were reduced by 90%
- Wet linen events were reduced by 78%
- Groin skin drier by an additional 2 hours and 35 minutes per day per participant
- Response time to incontinence products changes improved by 28%
- Average number of daily incontinence products per participant reduced from six to five
- Caregiver workload reduced by 19 hours per week
Some Notable Comments from Caregivers:
“...saved on clothing changes, which helped to prevent agitation in residents”
“...helps to establish a toileting plan by determining a pattern of when the residents void”
“...most residents did not find it intrusive or bothersome”
“...easy product to use”
“...improvement of sleep for the participants”
“...night time wetness checks were unnecessary”