Karen Winkowski, Ph.D, Ashland Global Director, Preservatives & Biomaterials09.20.19
The microbial quality and safety of wet wipes is of paramount importance for wet wipes producers to succeed in delivering innovative solutions with outstanding performance and integrity to consumers. Given the challenges formulators face with the current reduced pallet of preservative ingredients, this paper offers various preservation solutions to overcome these limitations and successfully capture the marketplace.
There are many new innovative technologies to enhance the functionality of wipes; many new solutions catering to the growing demand of eco-friendly, biodegradable, sustainable wipes; and recently, a variety of approaches to re-think wipes packaging to support a circular economy [1]. While all these great innovations are unraveling and expanding, the formulators and wipes producers are faced with less and less options as to how best to preserve these innovative wet wipes technologies. Regulatory restrictions and pressure from NGO on traditional preservative systems limit the palette of preservative solutions.
Quality and safety are paramount to the success of these new wet wipes technologies to deliver performance and integrity. One aspect of safety is that wet wipes need to remain microbial free before and during use. As most formulators know, wet wipes are an excellent breeding ground for the growth of many microorganism. The reduced pallet of preservatives is negatively impacting the microbial quality of the wet wipes and thus compromising consumer safety. Recently there have been several product recalls due to growth of undesirable microorganism in wipes [2][3].
Baby wipes, in particular, benefit from a properly formulated wipe as it can improve the skin health of babies [4]. Key to formulating baby wipes is to ensure that they are adequately preserved. The lack of a good preservation system in the wet wipes may result in a high risk of microbial contamination before or after use. Beyond the product having off-color, smell and different tactile attributes, using contaminated wipes can result in serious skin infections.
The list of controversial preservative ingredients must contend with constantly-changing global regulatory guidelines and consumer’s demands which are, in general, based on perceptions with little or no scientific basis. Phenoxyethanol is one such ingredients. While the Scientific Committee on Consumer Safety (SCCS) has concluded that phenoxyethanol is safe at use levels of 1% in cosmetics and the calculated Margin of Safety (MoS) also covers children and babies [5], some companies are still trying to find alternative preservation strategies to those based on phenoxyethanol preservatives.
Ashland’s solution to the shrinking list of preservative alternatives is to develop new technologies based on uncontroversial ingredients that can solve current needs for preservation solutions.
One such approach is the use of Optiphen DP or Optiphen DLP preservatives that are based on optimized delivery systems that maximize the efficacy of the actives at the oil/water interface, thus enhancing their bioavailability and in turn allowing the actives to work at lower levels while reducing the exposure to high level of preservatives [6]. These are new to the word combination without alcoholic antimicrobials based on skin friendly ingredients.
Another approach is to use functional materials such as Conarom b or Conarom p-2 aromatic which can offer a formulating strategy through the use of gentle fragrances that enhance consumer’s olfactory experience while delivering antimicrobial protection as a secondary effect [7].
Performance Data
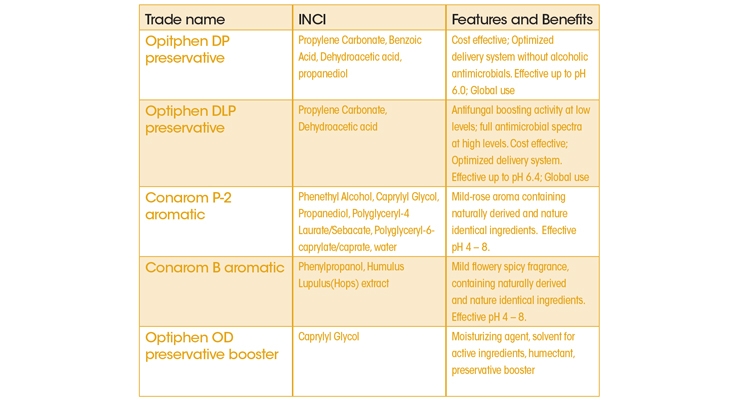
The performance of various preservatives solutions that are not based on Phenoxyethanol, was evaluated in formulated wipes system provided by different wipes producers. The description of Ashland’s preservative alternatives tested is shown in Table 1.
The various solutions were added to the wipes juice and mixed until uniform. The wipes juice was then added to a cellulosic nonwoven fabric at a ratio of 3:1 (liquid: nonwoven) and allowed to equilibrate for two days in the dark.
The wet wipes were then inoculated with a microbial suspension (about 107 cfu/ml) with a bacterial composite containing: Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli and Burkholderia cepacia or a mold composite containing Aspergillus brasiliensis and Candida albicans. To reduce the loss of humidity, the bag containing the inoculated wipes was heat sealed. The wipes were incubated at room temperature under dark conditions for the duration of the tests. At day seven, 14 and 21 after inoculation, the microbial content of the sample was measured by plate count.
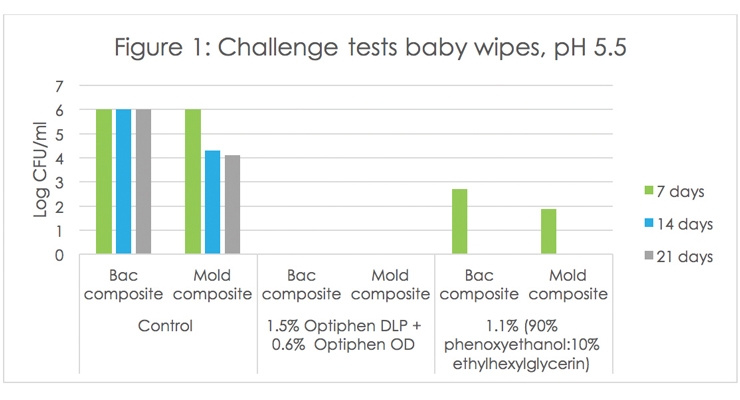
Figure 1 shows the results of a challenge test on baby wet wipes (pH 5.5) where the performance of non-phenoxyethanol based Ashland solutions was compared to a preservative based on phenoxyethanol. The control (unpreserved) was susceptible to the growth of bacteria and fungi. The addition of 1.5% Optiphen DLP preservative in combination with 0.6% Optiphen OD preservative booster resulted in no microbial recoveries, while the addition of a phenoxyethanol based preservative at 1.1% showed recoveries of both bacteria and fungi at day 7.
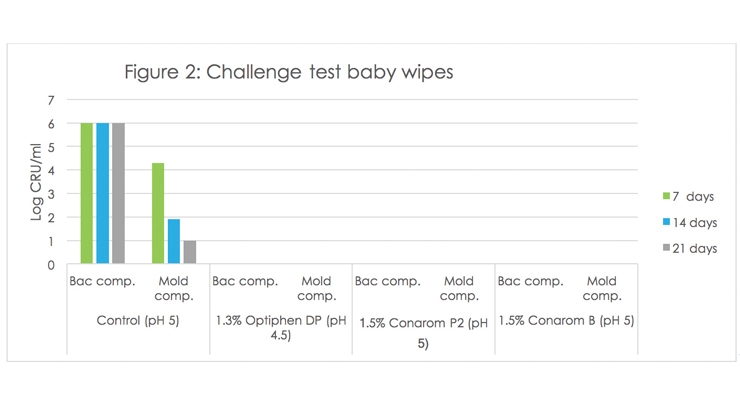
Figure 2 shows the results on baby wet wipes (pH 4.5 – 5.0). The addition of 1.3% Optiphen DP or the aromatics with antimicrobial properties Conarom P-2 or Conarom B (at 1.5%) offered also excellent microbial protection with no recoveries observed. The control was susceptible to microbial growth.
Conclusions
Working with optimized delivery systems and/or functional aromatic ingredients may offer alternatives to develop innovative wipe products with outstanding microbial quality and safety. Optiphen DP and Optiphen DLP preservative, Optiphen OD preservative booster, Conarom B and Conarom P-2 aromatic are innovative tools from Ashland that are available to solve alternative preservation challenges.
References
[1] Foschi E and Bonoli A. (2019): The commitment of Packaging Industry in the Framework of the European Strategy for Plastics in a Circular Economy. Adm. Sci 9,18: doi:10.3390/admsci901018.
[2] https://ec.europa.eu/consumers/consumers_safety/safety_products/rapex/alerts/?event=main.search&lng=en
[3] https://www.fda.gov/cosmetics/cosmetics-compliance-enforcement/cosmetics-recalls-alerts
[4] Vongsa R, Rodriguez K. Koening D and Cunningham C. (2019): Benefits of Using and Appropriately Formulated Wipe to Clean Deapered Skin of Preterm Infants. Global Pediatric Health 6:1-6. Doi:10.1177/2333794XI9829186. Journals.sagepub.com/home/gph
[5] Lilienblum, W. (2016): Opinion of the Scientific Committee on Consumer Safety (SCCS) – Final version of the opinion on Phenoxyethanol in cosmetic products. Regulatory Toxicology and Pharmacology. 82:156. https://doi.org/10.1016/j.yrtph.2016.11.007.
[6] Wingenfeld A. and Winkowski K (2016): Preservative technologies with optimized delivery systems. South African Pharmaceutical & Cosmetic review July 2016, pp. 18-20.
[7] Winkowski, K. and Wingenfeld A (2018): Functional Ingredient: An Alluring Subtle Fragrance with Antimicrobial Benefits. Household & Personal Care Wipes. Fall 2018, pp. 28 - 30
There are many new innovative technologies to enhance the functionality of wipes; many new solutions catering to the growing demand of eco-friendly, biodegradable, sustainable wipes; and recently, a variety of approaches to re-think wipes packaging to support a circular economy [1]. While all these great innovations are unraveling and expanding, the formulators and wipes producers are faced with less and less options as to how best to preserve these innovative wet wipes technologies. Regulatory restrictions and pressure from NGO on traditional preservative systems limit the palette of preservative solutions.
Quality and safety are paramount to the success of these new wet wipes technologies to deliver performance and integrity. One aspect of safety is that wet wipes need to remain microbial free before and during use. As most formulators know, wet wipes are an excellent breeding ground for the growth of many microorganism. The reduced pallet of preservatives is negatively impacting the microbial quality of the wet wipes and thus compromising consumer safety. Recently there have been several product recalls due to growth of undesirable microorganism in wipes [2][3].
Baby wipes, in particular, benefit from a properly formulated wipe as it can improve the skin health of babies [4]. Key to formulating baby wipes is to ensure that they are adequately preserved. The lack of a good preservation system in the wet wipes may result in a high risk of microbial contamination before or after use. Beyond the product having off-color, smell and different tactile attributes, using contaminated wipes can result in serious skin infections.
The list of controversial preservative ingredients must contend with constantly-changing global regulatory guidelines and consumer’s demands which are, in general, based on perceptions with little or no scientific basis. Phenoxyethanol is one such ingredients. While the Scientific Committee on Consumer Safety (SCCS) has concluded that phenoxyethanol is safe at use levels of 1% in cosmetics and the calculated Margin of Safety (MoS) also covers children and babies [5], some companies are still trying to find alternative preservation strategies to those based on phenoxyethanol preservatives.
Ashland’s solution to the shrinking list of preservative alternatives is to develop new technologies based on uncontroversial ingredients that can solve current needs for preservation solutions.
One such approach is the use of Optiphen DP or Optiphen DLP preservatives that are based on optimized delivery systems that maximize the efficacy of the actives at the oil/water interface, thus enhancing their bioavailability and in turn allowing the actives to work at lower levels while reducing the exposure to high level of preservatives [6]. These are new to the word combination without alcoholic antimicrobials based on skin friendly ingredients.
Another approach is to use functional materials such as Conarom b or Conarom p-2 aromatic which can offer a formulating strategy through the use of gentle fragrances that enhance consumer’s olfactory experience while delivering antimicrobial protection as a secondary effect [7].
Performance Data
The performance of various preservatives solutions that are not based on Phenoxyethanol, was evaluated in formulated wipes system provided by different wipes producers. The description of Ashland’s preservative alternatives tested is shown in Table 1.
The various solutions were added to the wipes juice and mixed until uniform. The wipes juice was then added to a cellulosic nonwoven fabric at a ratio of 3:1 (liquid: nonwoven) and allowed to equilibrate for two days in the dark.
The wet wipes were then inoculated with a microbial suspension (about 107 cfu/ml) with a bacterial composite containing: Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli and Burkholderia cepacia or a mold composite containing Aspergillus brasiliensis and Candida albicans. To reduce the loss of humidity, the bag containing the inoculated wipes was heat sealed. The wipes were incubated at room temperature under dark conditions for the duration of the tests. At day seven, 14 and 21 after inoculation, the microbial content of the sample was measured by plate count.
Figure 1 shows the results of a challenge test on baby wet wipes (pH 5.5) where the performance of non-phenoxyethanol based Ashland solutions was compared to a preservative based on phenoxyethanol. The control (unpreserved) was susceptible to the growth of bacteria and fungi. The addition of 1.5% Optiphen DLP preservative in combination with 0.6% Optiphen OD preservative booster resulted in no microbial recoveries, while the addition of a phenoxyethanol based preservative at 1.1% showed recoveries of both bacteria and fungi at day 7.
Figure 2 shows the results on baby wet wipes (pH 4.5 – 5.0). The addition of 1.3% Optiphen DP or the aromatics with antimicrobial properties Conarom P-2 or Conarom B (at 1.5%) offered also excellent microbial protection with no recoveries observed. The control was susceptible to microbial growth.
Conclusions
Working with optimized delivery systems and/or functional aromatic ingredients may offer alternatives to develop innovative wipe products with outstanding microbial quality and safety. Optiphen DP and Optiphen DLP preservative, Optiphen OD preservative booster, Conarom B and Conarom P-2 aromatic are innovative tools from Ashland that are available to solve alternative preservation challenges.
References
[1] Foschi E and Bonoli A. (2019): The commitment of Packaging Industry in the Framework of the European Strategy for Plastics in a Circular Economy. Adm. Sci 9,18: doi:10.3390/admsci901018.
[2] https://ec.europa.eu/consumers/consumers_safety/safety_products/rapex/alerts/?event=main.search&lng=en
[3] https://www.fda.gov/cosmetics/cosmetics-compliance-enforcement/cosmetics-recalls-alerts
[4] Vongsa R, Rodriguez K. Koening D and Cunningham C. (2019): Benefits of Using and Appropriately Formulated Wipe to Clean Deapered Skin of Preterm Infants. Global Pediatric Health 6:1-6. Doi:10.1177/2333794XI9829186. Journals.sagepub.com/home/gph
[5] Lilienblum, W. (2016): Opinion of the Scientific Committee on Consumer Safety (SCCS) – Final version of the opinion on Phenoxyethanol in cosmetic products. Regulatory Toxicology and Pharmacology. 82:156. https://doi.org/10.1016/j.yrtph.2016.11.007.
[6] Wingenfeld A. and Winkowski K (2016): Preservative technologies with optimized delivery systems. South African Pharmaceutical & Cosmetic review July 2016, pp. 18-20.
[7] Winkowski, K. and Wingenfeld A (2018): Functional Ingredient: An Alluring Subtle Fragrance with Antimicrobial Benefits. Household & Personal Care Wipes. Fall 2018, pp. 28 - 30